For many women with rheumatic disease, pregnancy is safer when she takes pregnancy-compatible medications. Use the guidance from the ACR Reproductive Health Guidelines included in the Discussion Guides to make informed decisions with your patients about medications in pregnancy.


Pregnancy Compatible Medications – Strongly Recommended
The following medications are strongly recommended for use in pregnancy:
- Azathioprine
- Certolizumab
- Colchicine
- Hydroxychloroquine
- Low-dose aspirin
- Prednisone (use sparingly)

Pregnancy Compatible Medications- Conditionally Recommended
These medications are considered safe in pregnancy with the included guidance:
- Cyclosporin/Tacrolimus: monitor blood pressure
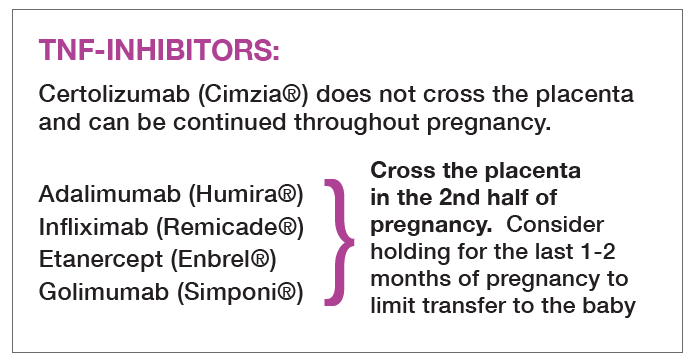
- Infliximab, Etanercept, Golimumab, Adalimumab: discontinue several half-lives prior to delivery
- Rituximab: only in very active rheumatic disease
Following the publication of the ACR Reproductive Health Guidelines in 2020, the FDA issued a Black Box Warning against the use of NSAIDs in pregnancy.
- NSAIDs (Meloxicam, Ibuprofen, Naproxen, etc.): consult with obstetrician before use in pregnancy

Insufficient Information for Use in Pregnancy
The following medications have insufficient information available about their safety for use in pregnancy:
Biologics
- Abatacept
- Anakinra
- Apremilast
- Avacopan
- Secukinumab
- Ustekinumab
- Tocilizumab
Small-Molecule Drugs
- Baricitinib
- Tofacitinib
- Upadacitanib
- any other newly approved biologics
- and other new approved small molecule medications

Medications that Are Incompatible with Pegnancy
The following medications should be avoided during pregnancy:
- Cyclophosphamide
- Methotrexate
- Mycophenolate Mofetil
- Thalidomide
- Lenalidomide
Biologic Medications in Pregnancy
Most biologic medications are IgG1 antibodies, which are the primary antibodies that cross the placenta. They do not cross, however, until about 16 weeks gestation. This likely prevents biologic medications from crossing the placenta during the first trimester, which is when organogenesis takes place and most birth defects occur. IgG1 antibodies cross at increasing levels as the pregnancy nears term. The baby, on the day of birth, has 60% higher concentrations of maternal antibodies than the mother. Therefore, for biologic medications that are IgG1 antibodies, the baby is likely to have MORE of the drug at birth than the mother.

When and whether to hold biologic medications prior to delivery is up for debate! The ACR Reproductive Health Guidelines recommend holding the TNF-inhibitors that are antibodies (adalimumab, etanercept, infliximab, and golimumab) several half-lives prior to delivery, so around 30-34 weeks gestation. In Europe, many experts recommend holding biologic medications earlier to ensure the baby is born without any of the medication. For women that would suffer significant harm from holding a biologic for several doses, for example a woman with severe inflammatory bowel disease, it may be reasonable to continue the drug through delivery (Mahadevan 2019).
Certolizumab is pegylated and is not a full antibody. By not containing an Fc portion, it cannot be transferred across the placenta. Babies exposed to certolizumab near delivery have undetectable or extremely low levels of certolizumab at birth (Mariette 2017). Therefore, the ACR Reproductive Guidelines strongly recommend use of certolizumab throughout pregnancy.